yoga book / physiology of movement / nervous system
See also the page with nerve maps
Contents
- 1 Nervous system
- 2 Nerve
- 3 Divisions of the nervous system
- 4 Terms and parts of the nervous system
- 5 Some terms
- 5.1 Allodynia
- 5.2 excitatory
- 5.3 hyperalgesia
- 5.4 Hyperpathy
- 5.5 inhibitory
- 5.6 Causalgia
- 5.7 Paraesthesia
- 5.8 Hypaesthesia
- 5.9 Blood-brain barrier
- 5.10 Cauda equina
- 5.11 Dermatoma
- 5.12 Golgi tendon organ
- 5.13 Cerebral membranes
- 5.14 Cranial nerves
- 5.15 Medulla oblongata
- 5.16 Intramuscular coordination
- 5.17 sciatic nerve
- 5.18 Pattern muscles
- 5.19 Motoneuronen
- 5.20 Myotome
- 5.21 Nerve compression syndrome
- 5.22 Nerve root
- 5.23 Nerve root compression syndrome
- 5.24 neuroradicular
- 5.25 Brachial plexus
- 5.26 Lumbar plexus
- 5.27 Sacral plexus
- 5.28 Proprioception
- 5.29 Proprioceptors
- 5.30 Pyramidal tract (tractus pyramidalis)
- 5.31 Pyramidal tract signs
- 5.32 spinal cord
- 5.33 Spinal canal
- 5.34 Spinal nerve
- 5.35 Cerebrospinal fluid
- 5.36 Parasympathetic tone
- 5.37 Sympathetic tone
- 5.38 Vagus nerve (N. vagus)
- 5.39 vagotone
- 6 Pain.
- 7 Pain history (short form)
- 8 Characteristic pain
- 8.1 Start-up pain (running-in pain, running-out pain)
- 8.2 Stress-induced pain
- 8.3 Painfulness on exertion
- 8.4 Pain in motion
- 8.5 Painfulness of movement
- 8.6 Stretching pain
- 8.7 Stretch pain
- 8.8 Pressure pain
- 8.9 Pressure pain / pressure dolence
- 8.10 functional pain
- 8.11 ischaemic pain (ischaemic pain)
- 8.12 Tapping pain
- 8.13 Inguinal pain
- 8.14 Afterload pain (pain reverberation)
- 8.15 Night pain
- 8.16 Pseudoradicular pain
- 8.17 Pulse-synchronised pain (throbbing, knocking)
- 8.18 Radicular pain
- 8.19 rest pain
- 8.20 Tension pain
- 8.21 Structural pain
- 8.22 Growing pains
Nervous system
A nervous system consists of nerve cells (neurons) and supporting and insulating glial cells. It does not necessarily have to have a brain, as in humans. The task of the NS is to register changes in the external and internal world, relate them to each other and compare them with previous ones in order to trigger reactions if necessary.
The simplest form is a diffuse nervous system or nerve network consisting of many individual nerve cells (neurons) without coordination centres for central control. Excitation is partially conducted in both directions of a nerve. In the course of the higher development of creatures, ganglia (nerve nodes in the PNS, one and a half dozen different types are known in mammals) are added, which can be divided into sensory and autonomic ganglia, and later brains. All vertebrates have a brain and a spinal cord running through the vertebrae, which together form the central nervous system CNS. The rest of the NS is referred to as PNS, where nerves need a connective tissue sheath. The human brain is not only THE nerve centre of the body, but with the hypothalamus (located under the thalamus) also has one of the most important glands of global control significance.
Nerve impulse
Nerve impulses are used for very short-term control. Unmyelinated (pithless, „uninsulated“) nerve fibres only conduct at a speed of around 1m/s (3.6 km/h), thick myelinated fibres can conduct at speeds of up to 100m/s (360 km/h). In general, a speed of 0.2 to 120 m/s is assumed for all mammals. In contrast, the effect of hormones via the bloodstream is slower. In the fastest blood vessels, such as the carotid artery, the speed is around 1 m/s and therefore on a par with the slowest nerves. In the small tissues of the thyroid gland, for example, the blood only flows at 3-5 cm/s, i.e. just around 0.15 km/h.
Nerve fibre
A nerve fibre is the elongated extension of a nerve cell and its sheath formed from glial cells. Extensions with sheaths are also referred to as axons. Thicker axons conduct faster than thinner ones. Vertebrates such as humans can have axons with several layers of insulating glial cells that conduct particularly quickly, this sheathing is called myelin sheath. The high nerve conduction speed is a necessity for fast movements of large bodies due to their long nerve tracts. Squids, whose movement apparatus manages without myelin sheaths, need 100 times thicker nerves with giant axons, which only reach a conduction speed of around 60 m/s. The nerve propagation time is not temperature-independent, the speed increases (in the physiological range) by 1-2 m/s per degree. Myelinated nerve fibres are usually those that transmit sensory or motor stimuli over longer distances and have something to do with a person’s relationship with the outside world.
Nerve
The term nerve refers to a large number of nerve fibres encased in additional connective tissue sheaths. Innervation is the supply of organs or body parts by nerve fibres and their transmission of stimuli. A distinction is made between
– afferent (leading to the CNS) and efferent (leading away from the CNS) nerve fibres. A further distinction is made
– according to the type of myelination and thus nerve conduction velocity and
– according to effect: sensory (perception), motor (movement apparatus), vegetative (autonomic NS with the parts sympathetic, parasympathetic and enteric).
Sympathetic and motor NS are also referred to together as somatic NS (external perception and motor function). The sympathetic NS is ergotropic, i.e. it increases the ability to act in response to actual or perceived stress (fight or flight). The parasympathetic NS is trophotropic, i.e. it serves to build up the body’s own reserves during rest and recovery (rest and digest). The enteric NS can regulate the function of the digestive organs completely autonomously, but is influenced by the sympathetic and parasympathetic nervous systems, which place their function in the current context of the overall system. It runs through almost the entire gastrointestinal tract. In humans, it has five times more neurones than the spinal cord.
motor endplate
A motor endplate is a synapse at which a motor nerve fibre chemically transmits its potential to a muscle fibre by means of neurotransmitter acetylcholine. The acetylcholine is transferred from the presynaptic part of the sarcolemma of the nerve cell via the synaptic cleft to the postsynaptic part of the sarcolemma of a muscle fibre. The motor end plate consists of the end button (presynaptic termination), the synaptic cleft of 10-15 µm width and the membrane section of the muscle cell. Both this membrane section and the terminal button are strongly unfolded and behave in a complementary manner. The action potentials of the axon cause the opening of voltage-gated Ca channels, and the released Ca mobilises synaptic vesicles containing the neurotransmitter acetylcholine, which is then emitted into the synaptic cleft and taken up by receptors of the postsynaptic membrane. While the distribution in the cleft occurs by diffusion, the vesicle transport in the terminal head of the nerve cell alone consumes energy. The binding of acetylcholine to the corresponding receptors leads to the opening of (cat) ion channels in the muscle cell, which causes a current flow that ultimately leads to the widespread release of calcium ions in the muscle cell, which reach each sarcomere of the myofibril, leading to the release of binding sites for the motor protein myosin on the actin filaments and thus to contraction of the muscle cell. The acetylcholine that is not used in the joint space is broken down by the enzyme acetylcholinesterase and the choline component is reabsorbed by the presynaptic membrane and recycled. After decomposition, it is ineffective at the postsynaptic membrane. The choline can be reutilised by the presynaptic membrane through the choline reuptake transporter. Disruption of the acetylcholine receptors, for example by autoantibodies, leads to disorders such as myasthenia gravis.
With a few exceptions, the muscles of the movement system are not controlled directly by the brain but via two motoneurons, the UMN and the LMN, also known as the 1st motoneuron and 2nd motoneuron.
The transmission of nerve impulses is based on action potentials. These are temporary deviations of the potential of the cell membrane from its resting potential. Nerve and muscle cells can form action potentials and conduct this excitation. Transmission between nerve cells usually occurs via neurotransmitters, as well as from nerve to muscle cells. In addition to excitatory potentials, inhibitory potentials are also possible. Muscle cells often interact directly electrically.
motor unit
A motor unit is the totality of a motoneuron with the muscle fibres innervated by it and thus the smallest functional unit of voluntary and involuntary motor activity. The smallest motor units in humans have 100-300 muscle fibres (muscles of the eye and fingers), the largest up to 2000 (quadriceps). The size of the nucleus of the motoneuron, the thickness of the axon and the number of muscle fibres largely correspond. The muscle fibres of a motor unit do not lie next to each other, but are distributed over the muscle on a cross-section of about 1 cm². Small motor units are recruited for low force exertion such as fine mechanics, while large motor units are recruited for larger forces. For maximum exertion of force, the neuronal impulse frequency of the acquired motor units is increased so that the individual twitches of the muscle fibres overlap and the resulting force adds up.
neuroendocrine system
For a long time, it was assumed that the nervous system worked with
neurotransmitters and gap junctions, while the endocrine system was a largely separate system that secreted hormones into the blood. The fact that neurones not only produce and release neurotransmitters, but can also release hormones, and on the other hand neurotransmitters activate hormone-producing cells, suggests a more general view and leads to the concept of the neuroendocrine system. These are all anatomical structures (cells, organs, organ tissue) that process or secrete neurohormones. In humans, the hypothalamic-pituitary system is a neuroendocrine system with the neurohypophysis as the neurohemal organ into which the hypothalamus releases hormones with the axons of its neurosecretory neurons. The human neuroendocrine system includes the hypothalamus-pituitary system epiphysis parathyroid gland adrenal medulla pancreatic islets C-cells of the thyroid gland paraganglia The diffuse neuroendocrine system (DNES, also known as the AUPD system, Amine Precursor Uptake and Decarboxylation) with various cells in the GIT, pancreas, UGT, bronchial system and other organs. While higher animals have the hypothalamus-pituitary system, insects, for example, have a different neurohemal organ.
Divisions of the nervous system
The nervous system can be categorised in different ways:
by location: peripheral and central nervous system
Peripheral nervous system (PNS)
the part of the nervous system that is not part of the CNS, i.e. is not surrounded by bone.
Central nervous system (CNS)
the part of the nervous system that is surrounded by bone, i.e. the brain and the part of the spinal nerves that runs in the spinal canal. After exiting via the intervertebral foramen, the corresponding parts of the spinal nerves as well as all peripheral nerves belong to the PNS.
after voluntary/involuntary control (somatic/autonomous)
Somatic nervous system / voluntary nervous system / animal nervous system / cerebrospinal nervous system
The somatic nervous system is the part of the nervous system that enables conscious control of the body via the muscles (voluntary motor skills) and contains its conscious sensory system, both for its own body and for the environment. It therefore contains both an afferent and an efferent part. The afferents form the ascending projection trajectories and lead to the projection centres and thus indirectly also to the association centres. The
afferents include in particular the nerves of the senses. The efferents exclusively supply striated muscles, including the motor parts of the cranial nerves (e.g. eye movement and facial expressions). eye movement and facial expressions) and, via the pyramidal tracts (PS, pyramidal system, also: corticospinal tract), the rest of the skeletal musculature. Another part, the extrapyramidal system (EPS), carries out largely automated movement patterns, which it adopts from the many repeated patterns of the PS. The pyramidal muscles have two serial motoneurons that are controlled by the
cranial nerves: The upper motor neuron (UMN) and the
lower mononeuron (LMN). The cell bodies (Betz giant cells) of the
UMN are located in the motor cortex in the brain, the associated
axons in the pyramidal tract. The UMN never control the muscles directly, but always via the LMN. The cell bodies of the LMN are located in the anterior horn of the grey matter of the spinal cord and form the motor core column in the entire spinal cord. In each vertebral segment, axons emerge as part of the spinal nerves and branch out from there to the motor endplates of the muscles of the associated myotome.
Vegetative nervous system
The autonomic nervous system is the part of the nervous system that controls the body involuntarily. It contains both an afferent and an efferent part. Direct, voluntary control is not possible, at best indirect influence is possible. Respiration, digestion, metabolism and heartbeat are subject to the autonomic nervous system, whereby the lungs are an example of organs that can be influenced by both parts of the nervous system. The sexual organs, endocrine and exocrine glands and the vascular system are also partially or completely subject to the autonomic nervous system. It can be subdivided into:
- Sympathetic nervous system / Sympathetic nervous system Parasympathetic
- Nervous system / Parasympathetic nervous system, of which the
N. vagus is of outstanding importance
The enteric nervous system is an independent regulatory system of the digestive tract that reacts to signals from the sympathetic nervous system or n. vagus. The ergotropic sympathetic nervous system and the trophotropic parasympathetic nervous system act partly antagonistically, partly synergistic. The most important regulatory centres of the autonomic nervous system are located in the brain stem and the hypothalamus, which establish various control circuits. Physical activity, but to a lesser extent even the idea of it, has an indirect influence on the autonomic nervous system as an arbitrary activity in terms of its extent and nature. Efferent motor nerve fibres of the autonomic nervous system innervate only smooth muscles with the exception of the influence on skeletal muscles. The digestive system is the largest autonomic organ system. The conceptual counterpart to the autonomic (vegetative) nervous system is the somatic/animal nervous system, which enables conscious control of the body and contains its conscious sensory system. Both parts of the nervous system are represented in CNS and PNS.
According to orientation of the line: afferent/efferent
afferent nerves / afferent
Afferent nerves are nerves that conduct sensory stimuli (sensory organs, but also proprioception) to the CNS (brain and spinal cord).
Relating to a neuron itself are the dendrites afferent and the neurites efferent.
efferent nerves / efferent
efferent nerves are nerves that conduct information from the CNS to the periphery (nerves, organs), in particular efferent fibres are the axons of the neurons.
Relating to a neuron itself are the dendrites afferent and the neurites efferent.
by gross influence on the body: sympathetic/parasympathetic
Sympathetic nervous system
The sympathetic nervous system is the part of the autonomic nervous system that controls the increase in performance and mobilisation of energy reserves. These effects are known as ergotropic. It is largely antagonistic to the parasympathetic nervous system. In the heart, the sympathetic nervous system is positively chronotropic, positively dromotropic (accelerating conduction), positively inotropic (increasing contractility), positively bathmotropic (lowering the stimulus threshold), positively lutitropic (promoting relaxation) and thus increases cardiac output in every possible way. In the blood vessels it has a vasoconstrictive effect, dilates the bronchi and inhibits mucus production and liquefies mucus, in the gastrointestinal tract it reduces gland secretion and peristalsis, in the UGT it tightens the bladder sphincter and weakens the detrusor vesicae muscle, in the eye it causes mydriasis (dilation of the pupil) and generally leads to increased sweat secretion. Increased excitation of the sympathetic nervous system is known as sympathetic tone and has a attenuating effect on HRV.
Parasympathetic nervous system
The parasympathetic nervous system is the part of the autonomic nervous system that is responsible for regeneration and building up energy reserves and is therefore largely the counterpart to the ergotropic sympathetic nervous system, which controls the increase in performance and mobilisation of energy reserves. These effects of the sympathetic nervous system are referred to as ergotropic. It is therefore largely antagonistic to the parasympathetic nervous system. The nerve fibres of the parasympathetic nervous system include some of the cranial nerves III (oculomotor nerve), VII (facial nerve), IX (glossopharyngeal nerve) and especially X (vagus nerve). The trigeminal nerve also carries sections of parasympathetic fibres, but these originate from the facial nerve. The parasympathetic nervous system has a negative chronotropic and negative dromotropic effect on the heart (promoting excitation conduction), a vasodilatory effect on the vessels in the genital area and a constrictive and mucus secretion-promoting effect in the bronchi, increases secretion and peristalsis in the digestive tract, promotes micturition in the UGT, promotes contraction of the uterus, promotes miosis (pupil constriction) and accommodation and promotes saliva production. Increased excitation of the parasympathetic nervous system is referred to as parasympathetic tone or usually as vagal tone; it has an HRV-enhancing effect.
Terms and parts of the nervous system
Reflexes
A reflex is a neurally mediated, involuntary, rapid, uniform reaction of an organism to a certain stimulus. The simplest case is the simple monosynaptic reflex arc (self-reflex: Receptor and effector are located in the same organ), in which a receptor (afferent, sensory cell) is directly connected to the effector (efferent, e.g. muscle, gland).e.g. muscle, gland) directly via a synapse in the anterior horn of the spinal cord. Examples of an own reflex are the patellar tendon reflex and Achilles tendon reflex. The terms are misleading because the reflex-triggering receptor is not located in the tendon but in the muscle, so it should be called quadriceps reflex and triceps surae reflex. In the case of polysynaptic reflexes, the receptor and effector are usually spatially separated, which is why they are also referred to as foreign body reflexes. Example: The cough stimulus triggered by a foreign body in the throat (pressure sensors), which innervates the expiratory musculature (effector). Reflex arcs are the simplest special cases of neuronal excitation circuits, as they are common in the vegetative and animal nervous system.
Intrinsic reflex
An intrinsic reflex is a monosynaptic reflex in which the sensor and effector are located in the same organ and is therefore the opposite of an external reflex. In most cases, the sensor in an intrinsic reflex is the muscle spindle and the effector is the muscle itself. The intrinsic reflex adjusts the muscle tension by counteracting strong stretch stimuli with a contraction impulse. To do this, the sensory stimulus is transmitted from the posterior horn of the spinal cord directly monosynaptically to the motoneuron, which leaves the spinal cord via the anterior root and triggers an action potential in the muscle. Intrinsic reflexes cannot be changed, i.e. they can be reduced through training. Known intrinsic reflexes are
- Achillessehnenreflex
- Adduktorenreflex
- Bauchdeckenreflex (Rectus abdominis)
- Bizepssehnenreflex
- Bizeps-femoris-Reflex
- Extensor-digitorum-Reflex
- Fingerbeuger-Reflex
- Masseterreflex
- Patellar tendon reflex (PSR)
- Pectoralis reflex
- Pronatorsreflex
- Radius periosteal reflex
- Scapulohumeral reflex
- Tibialis-posterior reflex
- Triceps tendon reflex
- Toe flexor reflex
External reflex
An extraneous reflex is one in which the sensor and effector are different. This means that extraneous reflexes cannot be monosynaptic.
monosynaptic reflex
A monosynaptic reflex is one in which the sensor and effector are the same muscle, so they are self-reflexes.
Nerve plexuses (plexus)
Somatic nerve plexus
– Plexus cervicobrachialis, which can be divided into
– – Plexus cervicalis for the musculature and sensitivity of the back of the head and neck, formed from the nerve roots C1 to C4
– – brachial plexus for the musculature and sensitivity of the shoulder and arm, formed from the nerve roots C5 to Th1
– lumbosacral plexus, which can be subdivided into
– – lumbar plexus for hip and leg, formed from the nerve roots Th12 to L4
– – sacral plexus formed mainly from the nerve roots L5 and S1, with motor and sensory fibres for the leg; most of the fibres of the sacral plexus unite to form the sciatic nerve
– – pudendal plexus with vegetative nerve fibres for the bladder, genitals and rectum, formed from the nerve roots S2 to S4
– coccygeal plexus controls urination and defecation except for the internal anal sphincter and the internal sphincter of the urinary bladder (these are controlled vegetatively). Neural roots S5, Co1, Co2
Important vegetative nerve plexuses
In the human body there are several nerve plexuses that are regionally responsible for the coordination of organ activities and information transmission. Sometimes the term plexus is used, sometimes the term ganglion.
– Plexus coeliacus in the upper abdomen, the largest paravertebral ganglion, left and right of the aorta at the level of the truncus aorticus (passage through the diaphragm) of the exit of the truncus coeliacus
– mesenteric plexus superior in the mid-abdomen
– mesenteric plexus inferior/caudalis in the lower abdomen
Further vegetative plexuses are found in the vicinity of organs and with a strong connection to them:
– external carotid plexus for the sweat glands, the smooth muscles of the hair follicles and blood vessels in the area of the head and face
– Internal carotid plexus for the eyes (pupillary dilator muscle, orbital muscle, Musculus tarsalis) and the lacrimal and salivary glands
– Plexus cardiacus at the heart from the terminal branches of the parasympathetic vagus nerve
– Plexus pulmonalis in the wall of the bronchi for bronchi and lungs
– Gastric plexus with parasympathetic fibres to supply the stomach
– Vesical plexus with parasympathetic fibres to supply the urinary bladder and genitals
– Pelvic plexus for the parasympathetic supply of the rectum (peristalsis) and the parasympathetic supply of the internal intestinal sphincter, which is not subject to voluntary control (relaxation)
– hypogastric plexus superior and hypogastric plexus inferior for the innervation of the sexual organs (seminal vesicles, prostate, vas deferens)
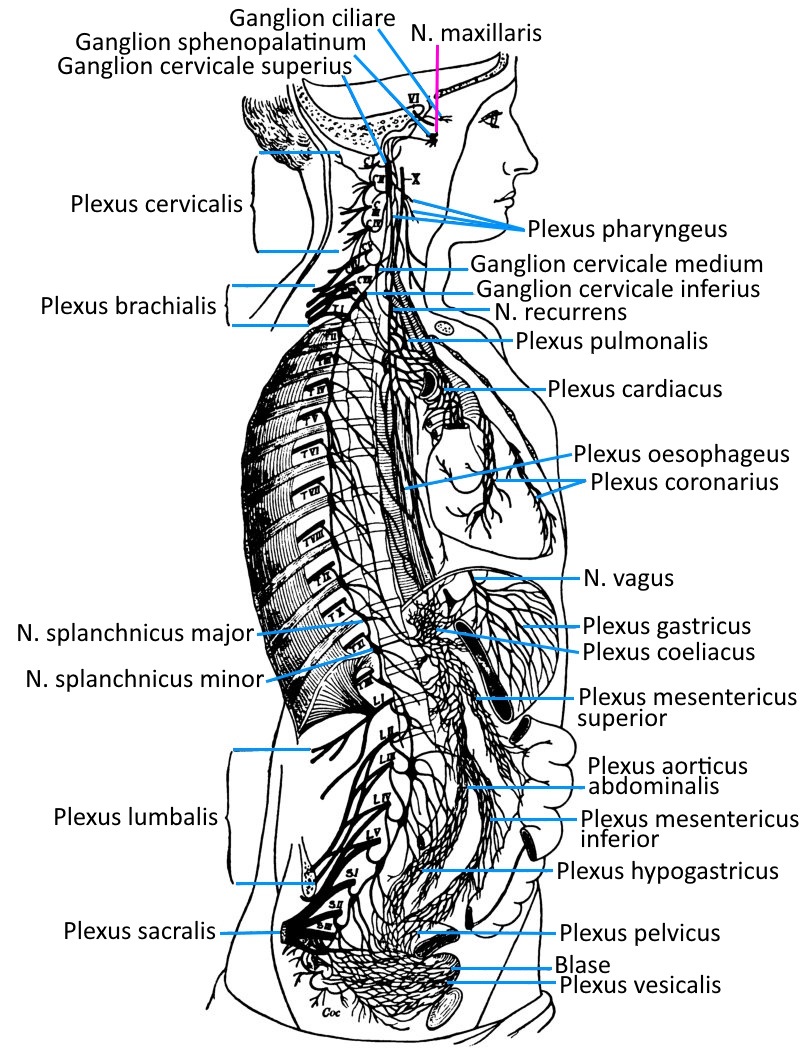
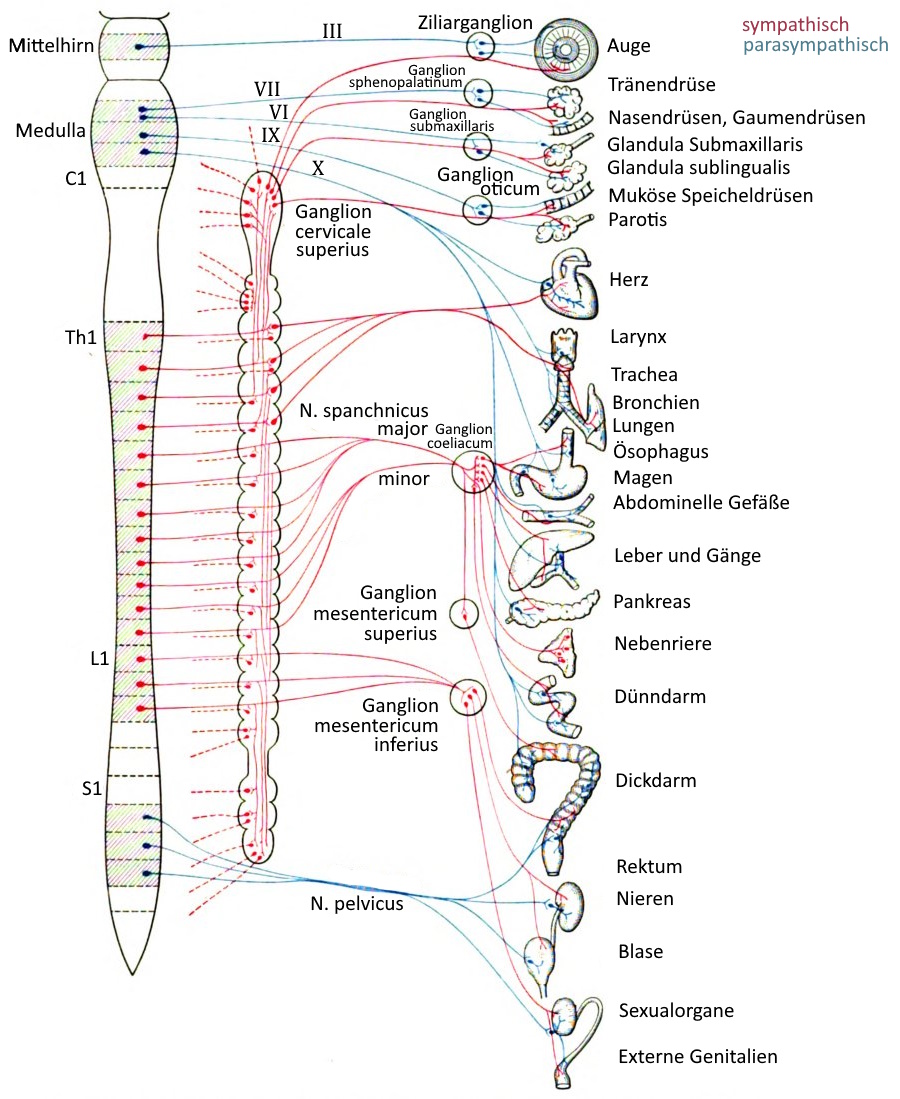
The intestinal nervous system, a collection of neurons distributed over the entire torso intestine, does not correspond to the morphological definition of a nerve plexus, but should nevertheless be regarded as a vegetative nerve plexus; These are primarily the submucosal plexus (Meissner’s plexus) in the tela submucosa of the large intestine and a myenteric plexus (Auerbach plexus) in the tunica muscularis, between the stratum circulare and stratum longitudinale of the smooth intestinal musculature.
Neuron
A nerve cell (neuron) is a cell that, in addition to the cell body (soma, perikaryon), usually also contains (possibly myelinated) efferent (cellulifugal) projections (axon) and unmyelinated afferent (cellulipetal) projections (dendrite).
Axon
The axon (neurite) is the extension of a nerve cell (neuron) that conducts signals away from the cell body of the neuron. A nerve fibre is the entirety of the axon and its sheath (axolemma)
dendrite
A dendrite is an extension of up to 100 µm in length that emerges from the cell body of a neuron and serves to (predominantly) absorb stimuli. It can become fibrous like a tree. Some dendrites have presynaptic specialisations that can form dendro-dendritic synapses. Electrical synapses (gap junctions) also occur between dendrites, as do bidirectional synapses (reciprocal synapses).
Synapse
A synapse is an anatomical structure that allows the transmission of a stimulus from one
neuron to another or to an effector (e.g. muscle, gland, sensory cell). The transmission can be chemical or electrical (
gap junction). In both cases, the synapse consists of:
- presynapse (transmitter)
- synaptic cleft
- Postsynapse (receiver)
In the case of the chemical synapse, the presynapse
releases neurotransmitters into the approx. 20-30 nm wide synaptic cleft (exocytosis), which dock onto receptors in the postsynapse. At the
gap junction (electrical synapse), the gap is only 3.5 nm wide so that ion channels (connexons) can conduct the stimulus without delay (basically bidirectionally). Gap junctions
are found particularly in very fast stimulus transmission. Synapses are divided into excitatory and inhibitory synapses, further classified by effector
- neuromuscular synapse ( motor endplate): with a muscle cell
- neuroglandular synapse: with a gland cell
- interneural synapse: between two nerve cells
- neurosensory synapse: with a secondary sensory cell
and contacts
- axo-somatic synapse: between axon and cell body
- axo-axonic (axo-axonal) synapse: between two axons
- axo-dendritic synapse: between axon and dendrite tree
- dendro-somatic synapse: between dendrites of one neuron and the body of another
- dendro-dendritic synapse: between dendrites of different neurons
- somato-somatic synapse: between cell bodies of a neuron and one of its immediate neighbours
- somato-axonal synapse: between nerve cell bodies and the axon of another neuron
- somato-dendritic synapse: between nerve cell body and dendrite of another neuron
The endowment of humans with synapses is not fixed and unchanging, but changes in the sense of continuous formation of new synapses (synaptogenesis), which is referred to as neuroplasticity, and continuous synapse elimination (elimination of unused synapses).
Gap junction
The gap junction is the electrical form of a synapse. Its gap is only 3.5 nm wide, so that ion channels (connexons) can conduct the stimulus without delay (basically bidirectional). Gap junctions are found above all in very fast stimulus transmission.
Neurotransmitters
Neurotransmitters are the substances released from the presynapse into the synaptic cleft during chemical synapses. Depending on the context, these are
- Adrenaline: when taken up by adrenoreceptors, it increases blood pressure, heart rate and arterial vascular tone. It has a catabolic effect on glycogen and glucose metabolism and causes the breakdown of triglycerides in fatty tissue.
- Acetylcholine: neurotransmitter of the CNS and PNS that binds to nicotinic and muscarinic receptors and is used in the motor endplate. Among other things, it causes muscle contraction, has a controlling effect in the central nervous system and is involved in memory and reward functions.
- Dopamine (prolactin inhibiting hormone, PIH): acts on adrenoreceptors or dopamine receptors (currently: D1 to D5), increases the effect of the sympathetic nervous system, increases blood flow to the abdominal and renal vessels to improve kidney performance, but also inhibits the release of prolactin as a neurohormone of the hypothalamus. Dopamine also has a disinhibitory effect (inhibits inhibition)
- in the cerebrum.
- Glutamine: is a non-essential amino acid and, with 20% of the free amino acids in plasma, a widely distributed protein in serum. Glutamine is blood-brain barrier-crossing and, when converted to glutamate, acts as an excitatory (stimulating) neurotransmitter.
- Glycine: as a neurotransmitter of the brain stem and spinal cord inhibits the glycine receptor, but stimulates the NMDA receptor alongside the main glutamate.
- GABA (?-aminobutyric acid): Neurotransmitter of the CNS with a mainly inhibitory effect on 3 different GABA receptors
- Serotonin: neurotransmitter of the PNS and CNS, involved in thermoregulation, pain perception, sleep control, eating and sexual behaviour, memory performance at 5-HT receptors
- Various peptides
Some terms
Allodynia
Allodynia is pain caused by an inadequate trigger, usually pain on touch. Increased sensitivity, for example in diabetic polyneuropathy or nerve damage from other causes, can lead to this symptom. In diabetes mellitus, for example, even touching a bedspread can lead to allopathic pain, not unlike the increased sensitivity in gout.
In migraine, allodynia is a sign of sensitisation of the trigeminal nerve network. Burning mouth syndrome is characterised by hyperalgesia and allodynia of the tongue and mouth. The hypersensitivity that occurs after shingles is also an allodynia. The sensitivity of the skin to touch after sunburn (insolation) is also an allodynia. However, repeated damage such as trauma can also lead to allodynic changes in pain perception.
excitatory
Term from neurophysiology: excitatory, stimulating. The opposite is inhibitory.
hyperalgesia
Hyperalgesia is an increased sensation of pain. Hyperalgesia can be primary, in which case it is peripherally mediated and limited to the site of damage, or secondary, in which case it is mediated by the CNS and the sensation of pain can extend beyond the site of damage. The primary form is characterised by hypersensitivity to heat due to a reduced stimulus threshold for nociception. The secondary form is mainly characterised by hypersensitivity to mechanical stimuli.
Hyperpathy
Hyperpathy is a central (thalamic) pain syndrome with sensory disturbance in the form of a locally increased pain threshold. The pain radiates and lasts for a long time.
The synaptic transmission is plastically altered, repetitive stimuli cause long-term potentiation, i.e. sensitisation of the pain pathways, which also explains the long reverberation of the pain.
inhibitory
Term from neurophysiology: inhibitory. The opposite is excitatory.
Causalgia
Causalgia (CRPS II, not to be confused with CRPS I, also known as Morbus Sudeck) is a pain syndrome, that occurs as a result of a lesion of a peripheral nerve with sympathetic dysregulation. The pain sensation is not necessarily limited to the site of the injury.
The classification of the International Association for the Study of Pain (IASP) describes causalgia as a diagnosis of exclusion when the two criteria are present at the same time:
- continuous pain, allodynia and hyperpathy due to nerve damage, whereby the pain can exceed the area of coverage
- Occurrence of oedema, altered skin perfusion or abnormal sweat secretion in the area of pain
Causalgia can manifest itself sensory, motor and autonomic and usually shows a stabbing, burning pain that is not necessarily limited to the area supplied by the nerve. Allodynia or hyperpathy can occur concomitantly, as well as atrophy, oedema, disorders of sweat secretion or thermoregulation as signs of autonomous damage. This can also include growth disorders of the hair or nails. Motor disorders can manifest as spasms, muscle weakness, dystonia or tremor.
Causalgia often affects the median and tibial nerves.
In contrast to causalgia, CRPS I (Morbus Sudeck) is not caused by direct nerve damage, but a previous immobilisation or trauma.
Paraesthesia
Unpleasant, irritating but not painful false sensation or discomfort without an adequate trigger, which is often subjectively described as burning, tingling, formication, pins and needles, a furry feeling, tingling, itching, swelling or cold or warm sensations. Hands, fingers and feet are most commonly affected. The paraesthesias can be divided into
- transient: temporary, caused by nerve compression, reduced blood supply, hyperventilation (due to alkalosis), migraine
- chronically persistent: irreversible nerve damage, polyneuropathy, e.g. in the context of diabetes mellitus (in 50% of cases); hypothyroidism (hypothyroidism), nerve congestion syndromes, further drug-induced paraesthesia
Despite paraesthesia, the skin’s sensitivity to touch may remain unchanged. This is caused by hyperexcitability of peripheral sensory receptors, nerve fibres or pathways of the CNS.
Hypaesthesia
Hypaesthesia is a sensory disorder with reduced pressure and touch sensation. It can be generalised or localised. It is not uncommon for hypaesthesia to occur together with paraesthesia. In the case of a disc hernia (intervertebral disc event), the relevant dermatome is affected; in the case of peripheral nerve damage, the nerve’s supply area is affected. In addition to spinal events, radiculopathies, circulatory disorders, the causes can also be poisoning, cerebral infarction or trauma affecting nerve plexuses.
Blood-brain barrier
The blood-brain barrier regulates the exchange of substances between the blood and the brain by preventing substances that should not enter the brain tissue from passing through the vascular membrane. The structures involved are: Capillary endothelium, basement membrane, astrocyte processes, microglia, pericytes. The capillary endothelium with its tight junctions, which minimise diffusion and also transport fewer vesicles, is particularly important. Transponders and receptors, on the other hand, ensure that important substances such as glucose, amino acids, nucleosides and some neurotransmitters can pass through. Fat-soluble substances can only pass through if their molecular weight is less than 500 Daltons. These include nicotine, alcohol, blood gases, but also narcotics such as halothane. Ions and polar substances such as glucose need special transporter systems to pass through the blood-brain barrier. This ensures that the composition of the interstitial fluid of the brain remains largely constant and appropriate. On the other hand, uncontrolled leakage of neurotransmitters from the brain into the blood is also prevented. The blood-brain barrier can be influenced to a certain extent and is susceptible to pathogenic processes. In diseases such as Parkinson’s, Alzheimer’s, Chorea-Huntington’s, epilepsy, headaches and MS, there are changes in the blood-brain barrier. An analogy can be found in the form of the blood-spinal cord barrier in spinal cord.
Cauda equina
from lat.: Ponytail refers to the end (Conus medullaris, approximately level L1) of the spinal cord in a ponytail-like manner towards the caudal in the direction of the sacrum of the nerve roots of the spinal nerves, which leave the spinal column through their corresponding intervertebral foramen.
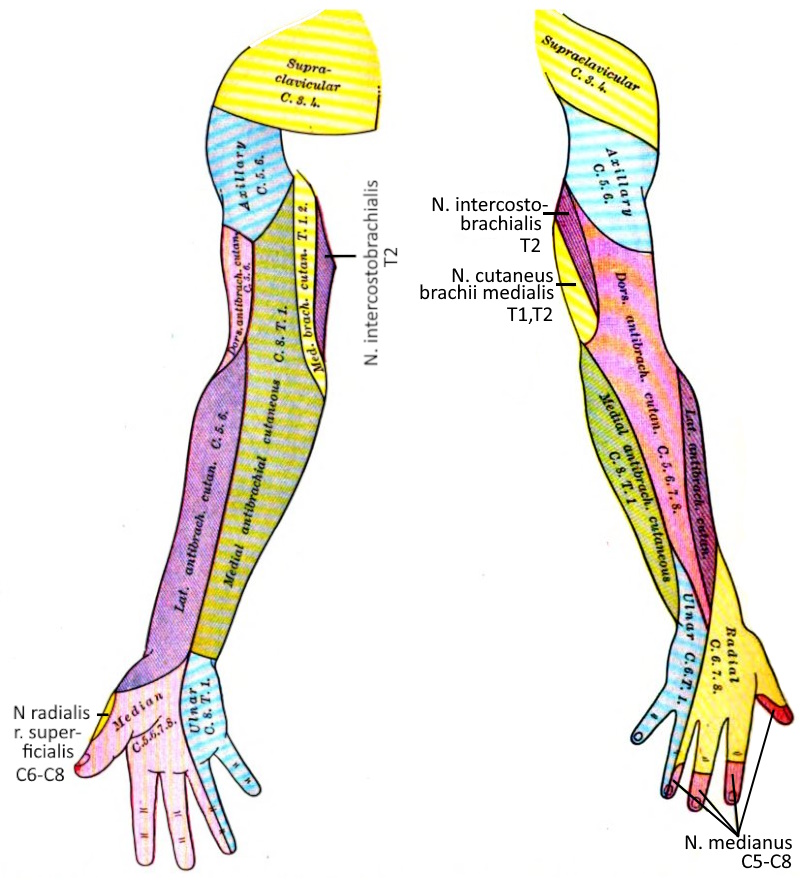
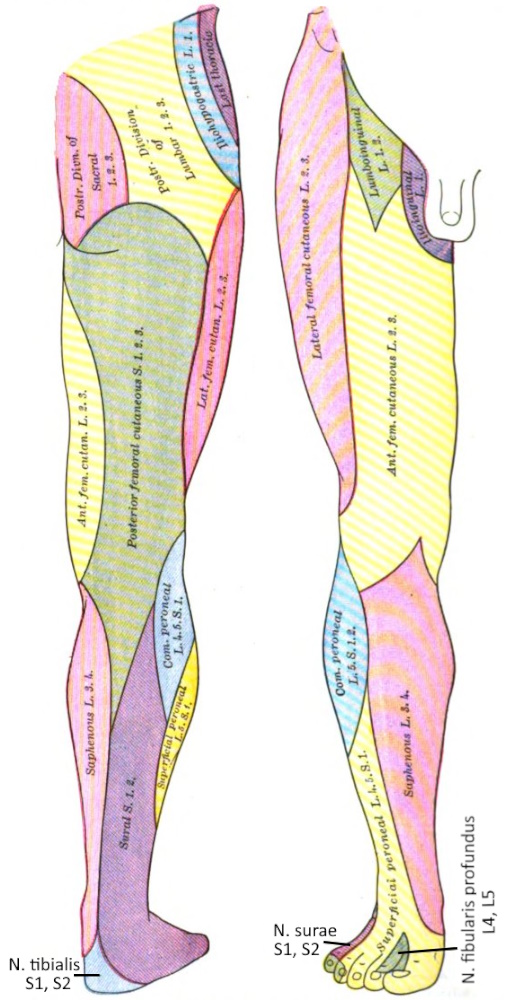
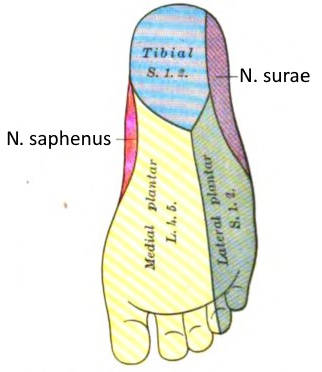
Dermatoma
A dermatome is the area of skin supplied by a spinal nerve.
See this image.
Golgi tendon organ
Nerve plexus at the transition of the contractile muscle belly to the tendon, which reports the state of tension (generally corresponds to the
tendon force of the muscle) to the CNS. In the sense of autogenous inhibition, the motor neuron of this muscle is inhibited, i.e. the stimulus for further contraction is attenuated, but at the same time the antagonist is stimulated via excitatory switching neurons (interneurons). Autogenous inhibition serves to regulate muscle tension and protect against overstraining. The Golgi tendon organ is one of the proprioceptors.
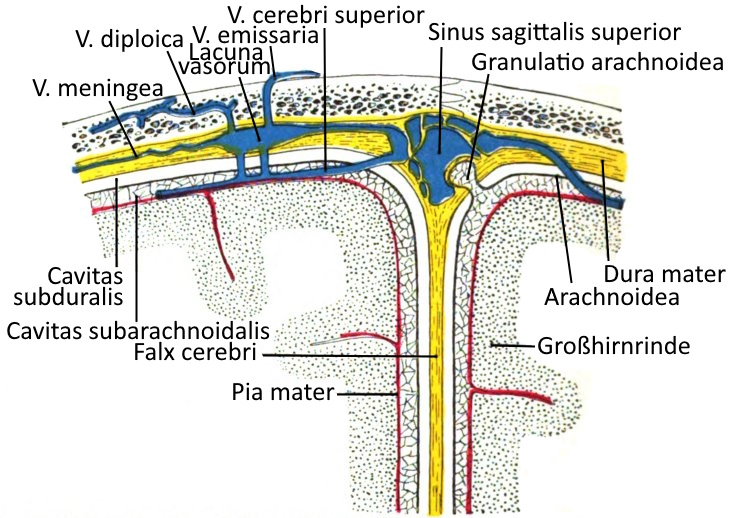
Cerebral membranes
The meninges are layers of connective tissue that envelop the entire brain and continue caudally of the foramen magnum as the spinal meninges: dura mater spinalis, arachnoid spinalis and pia mater spinalis.
The meninges from the outside (superficial) to the inside (profound):
Dura mater
The dura mater, which lies directly under the skull bone, consists of an outer leaf (stratum periostale) and an inner leaf (stratum meningeale). It contains the meningeal veins, the lacunae varosum and the sagittal sinus superior. The granulationes arachnoideae, superficially orientated protrusions of the arachnoid, protrude into the dura mater. In the area of the brain, the periosteum of the cranial bones is fused with the dura mater, in the spinal cord the epidural space lies in between. A subdural space between the dura mater and the next most prominent meninges, the arachnoid membrane, does not occur physiologically, only postmortem and under certain pathological conditions. The dura mater forms a median duplication directed into the brain, the falx cerebri, which divides the brain into its two hemispheres, as well as the falx cerebrelli, which divides the cerebellar hemispheres, and the tentorium cerebelli.
Arachnoid membrane
Between the middle of the classic three meninges, the arachnoid membrane, and the most profound meninges, the pia mater, which lies directly on the cortex, lies the subarachnoid cavity, the cavitas subarachnoidalis, which is filled with cerebrospinal fluid.
According to studies from 2023 by Møllgård et al., the subarachnoid space is divided into two domains by a thin membrane known as the subarachnoid lymphatic-like membrane. This membrane is thought to filter the cerebrospinal fluid and play a role in inflammatory reactions and defence against pathogens.
Pia mater
The pia mater is the most profound of the meninges and lies directly on the cortex. Between it and the more superficial meninges, the arachnoid membrane, lies the subarachnoid space filled with cerebrospinal fluid. The arachnoid membrane extends into the dura mater in the granulationes arachnoideae.
Cranial nerves
The cranial nerves emerge from the skull in pairs and conduct signals from or to the brain.
- I N. olfactorius conducts signals from the nose to the brain and enables smelling
- II N. opticus transmits signals from the eye to the brain and enables vision
- III N. oculomotorius controls eye movements, the eyelid retractor and the iris (iris)
- IV N. trochlearis controls the oblique upper eye muscle
- V N. trigeminal nerve is divided into ophtalmicus, maxillaris and mandibularis, transmits signals from the face to the brain and controls the masticatory muscles
- VI N. abducens controls the lateral eye muscle
- VII N. fascialis controls the mimic muscles and the stapedius muscle (middle ear), transmits taste sensory stimuli from the front two-thirds of the tongue to the brain, innervates all head glands with the exception of the parotid gland
- VIII N. vestibulococclearis transmits signals from the cochlea and the vestibular system to the brain
- IX N. glossopharyngeus innervates the pharyngeal muscles and controls swallowing, transmits sensory stimuli from the posterior third of the tongue to the brain
- X N. vagus most important nerve of the parasympathetic nervous system, controls many internal organs, including the heart
- XI N. accessorius controls the trapezius and the sternocleidomastoid
- XII hypoglossal nerve controls the movements of the tongue
The terminal nerve is sometimes referred to as the zeroth cranial nerve (presumably perception of pheromones) and the intermediate nerve, which belongs to the fascial nerve, as the 13th cranial nerve.
Medulla oblongata
The medulla oblongata (medulla oblongata: intracranial extension of the spinal cord) is the most caudal three-part part of the brain, which borders cranially on the pons and caudally on the spinal cord, with the exit of the 1st ventricle usually being the most important part. Spinal nerves is usually assumed to be the boundary. The medulla oblongata is the most important control centre for many vital functions, including parameters of blood circulation, respiration, sneezing, swallowing, sucking and coughing reflexes and vomiting (retroperistalsis). Some receptors for the regulation of the acid-base balance are also located here. Part of the pyramidal tract, in particular the decussatio pyramidum, is located in the medulla oblongata.
Intramuscular coordination
Intramuscular coordination is the interaction of innervation with the musculature and, alongside hypertrophy, is the most important factor in determining the maximum strength of a particular muscle. This factor is particularly important for athletes who want to increase their strength without gaining mass, such as endurance athletes (triathletes, runners) or athletes who do strength training. This is also important for other maximum strength sports such as weightlifting or speed-strength sports such as jumping disciplines. In specific training, work is carried out in the load range of 85-100% of the 1-RM with repetition numbers between 1 and 3 (3-6 sets).
sciatic nerve
The common fibular nerve and the tibial nerve are referred to as the „sciatic nerve“. Both emerge independently from the lumbosacral plexus, but pass through a common sheath. The sciatic nerve supplies the lower limb, whereby all flexors (exception: M. biceps femoris caput breve) of the knee joint and OSG are supplied by the tibial nerve and the stretcher and pronators of the jump joints from the common fibular nerve. If the nerve is irritated or compressed in its course, be it the underlying spinal nerves or the sciatic nerve in its course in the dorsal hip area, this leads to disorders such as sciatica or lumbalgia or lumbalgia. ischialgiforme or lumbalgiforme pain. However, these can also be caused simply by compression by a muscle, as in piriformis syndrome/deep gluteal syndrome DGS. Similar disorders also occur when the relevant nerves are still compressed in the spinal canal, such as in spondylolisthesis (spondylolisthesis) or spinal canal stenosis, so that these images must be differentiated by differential diagnosis. In some cases, this is already possible with the help of anamnesis and provocation tests, otherwise imaging such as MRI must be used. Irritation of the sciatic nerve can also be triggered by exposure to cold or pressure (sometimes associated with DGS). Similar pain occurs in facet syndrome, but it is pseudoradicular and not radicular and does not cause any muscular or sensory deficits.
Pattern muscles
Myofascial muscles are muscles that are tested in clinical testing as a proxy for a myotoma and a spinal nerve, for example if there is suspected damage to a WS-segment.
- C4: Diaphragm
- C5: Deltoideus, Infraspinatus, Supraspinatus, Rhomboiden.
- C6: Bizeps, Brachioradialis
- C7: Trizeps, Pronator Teres, Extensor carpi radialis, Flexor carpi radialis, Pectoralis major.
- C8: Interossei, Abductor pollicis brevis, Abductor digiti minimi, Flexor carpi ulnaris, Flexor pollicis brevis.
- Th10-Th12: Bauchmuskeln
- L1: Cremaster
- L3: Quadrizeps, Iliopsoas, Adductor longus, Adductor brevis, Adductor magnus.
- L4: Quadrizeps femoris: Vastus lateralis
- L5: Fibularis longus, Extensor hallucis longus, Tibialis anterior, Tibialis posterior, Gluteus medius.
- S1: Trizeps surae, Gluteus maximus
- S2: Flexor digitorum brevis
- S3/S4: Bulbospongiosus
- S4/S5: Sphinkter ani externus
Einige der Kennmuskeln sind mit sehr leichten Tests prüfbar:
- Quadrizeps: Kniebeuge
- Trizeps surae: Zehenstand
- Tibialis anterior: Fußballenstand
Motoneuronen
A motor neurone is an efferent nerve cell (neurone) in the anterior horn of the spinal cord, which directly or indirectly innervates a skeletal muscle or a gland with its axon. The motor neurones have the longest nerve fibres in the entire body and form the efferent nerve pathways. One classification of motor neurones is into visceral motor neurones (control the smooth muscles) and somatic motor neurones (control the striated muscles). Like many neurones, motor neurones have a soma (cell body), axons (transmitting) and dendrites (receiving). Most motor neurones have one axon and several dendrites. The axons are coated with myelin sheaths for fast signal transmission. The axon branches into a tree, the telodendron, which innervates various fibres in the muscle. For this purpose, axon terminal end buttons release the respective neurotransmitter, which is taken up by the motor end plates of the muscles. The motor neuron together with the associated muscle fibres is referred to as a motor unit. The conduction velocity of motor neurones is between 2-30 m/s (small gamma motor neurones) and 60-12 m/s (alpha motor neurones).
With a few exceptions, the muscles of the movement system are not controlled directly by the brain but via two motoneurons, the UMN and the LMN, also known as the 1st motoneuron and 2nd motoneuron. The upper motoneuron (upper motoneuron, UMN or 1st motoneuron) and the lower motoneuron (lower motoneuron, LMN or 2nd motoneuron). Motor neurones are nerve cells that exert direct or indirect control over a muscle. A distinction is made between somatic motor neurones (innervate skeletal muscles) and visceral motor neurones (innervate smooth muscles).
Disorders of the 1st motor neurone (UMN) usually lead to spastic paralysis, disorders of the 2nd motor neurone (LMN) to flaccid paralysis
UMN
Neurones of the cerebral cortex and brainstem that transmit their signals to interneurones and LMN. The cell nuclei of these UMN nerves are predominantly located in the motor cortex of the brain, its axons as various tracts in the pyramidal tract and the extrapyramidal motor tract to the LMN in the anterior horn of the grey matter of the spinal cord.
LMN
In each WS-segment, axons of the LMN leave the spinal nerves via the spinal cord and thus the WS. These run in several branches into its supply area (myotome: The musculature supplied by a spinal nerve and by analogy dermatome: the area of skin supplied by a sinal nerve). The LMN is the efferent branch of all movements and reflexes (see below) and directly innervates the muscles. The neurotransmitter between the UMN and LMN is glutamate.
Myotome
A myotome is the set of all muscles supplied by a spinal nerve.
Nerve compression syndrome
Syndrome with pain, sensitivity disorders such as numbness, tingling, reduced sensation and innervation disorders caused by pressure on a nerve. This can be a spinal cord that has just emerged from the spinal nerve, as in disc injury, then this is referred to as nerve root compression syndrome or a radiculopathy (lat „radix“: root) or a nerve lying further in the periphery, for example when tendons swell in the area of a physiological constriction between bones and press on a nerve, as is the case with cubital tunnel syndrome (affected: ulnar nerve), cubital tunnel syndrome (affected: ulnar nerve). ulnaris), tarsal tunnel syndrome (affected: tibial nerve) and carpal tunnel syndrome (affected: median nerve). Another common nerve compression syndrome is Morton’s neuroma, which usually occurs as part of spread foot and causes metatarsalgia.
Nerve root
Nerve roots are the segmental parts of the spinal cord that merge and emerge from the spinal nerves. Pressure on these can lead to nerve root compression syndrome and, later in the course of the nerve, to nerve (root) compression syndrome.
Nerve root compression syndrome
A spinal nerve compression syndrome caused by pressure on a spinal nerve exiting the spinal cord. See there for description.
neuroradicular
Complaints triggered by nerve root compression syndrome of one or more nerve roots emerging from the spinal column, In short, the term radicular is also used, see also the explanations there.
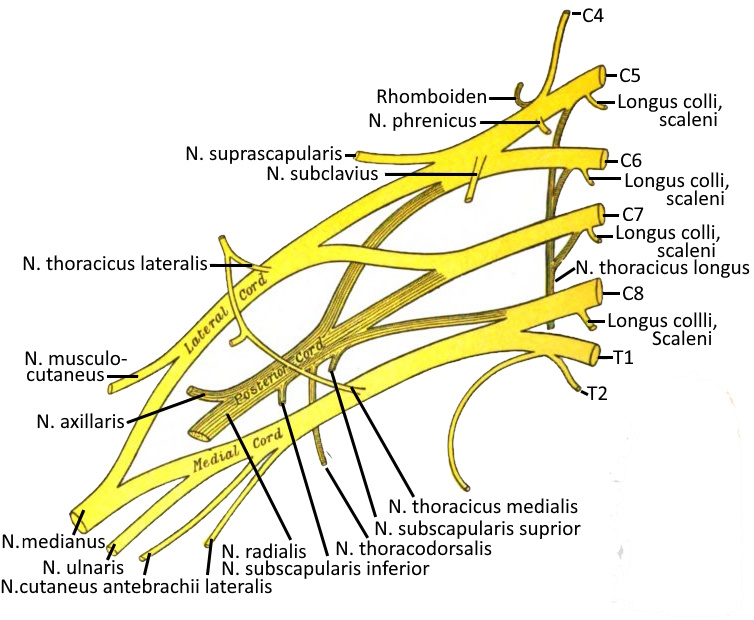
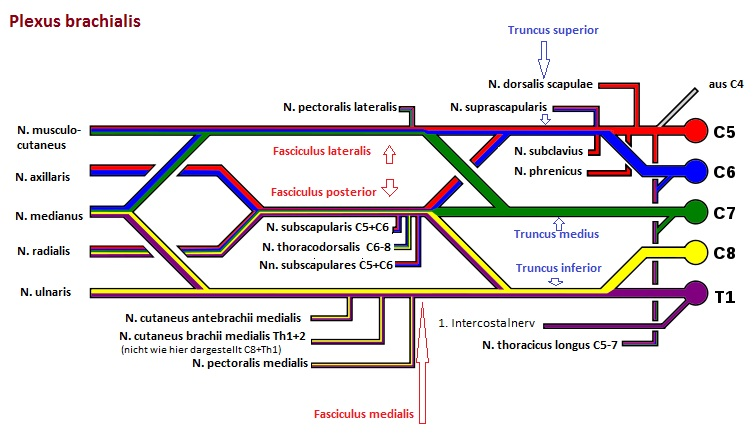
Brachial plexus
The brachial plexus (brachial plexus) is part of the PNS and is a nerve plexus consisting of the ventral branches of the spinal nerves of the last four cervical spine segments and the first thoracic spine segment (C5-Th1) as well as smaller bundles of C4 and Th2. Compression of the brachial plexus can lead to thoracic outlet syndrome.
Supraclavicular originate from truncus superior (C5, C6), truncus medius (C7) and truncus inferior (C8, Th1): Rami musculares, N. suprascapularis, dorsalis scapulae nerve, long thoracic nerve, subclavian nerve.
The largest part of the brachial plexus lies infraclavicular, where the lateral fasciculus, medial fasciculus and posterior fasciculus arise:
medial pectoral and lateral pectoral nerves. pectoralis lateralis (fasciculus lateralis or medialis), N. musculocutaneus (fasciculus lateralis), N. medianus (fasciculus lateralis), N. medianus (lateral and medial fasciculus), ulnar nerve (medial fasciculus), medial cutaneous nerve. cutaneus brachii medialis (Fasciculus medialis), N. cutaneus antebrachii medialis (Fasciculus medialis), N. axillaris (Fasciculus posterior), N. radialis (fasciculus posterior), N. subscapularis (fasciculus posterior), N. thoracodorsalis (fasciculus posterior)
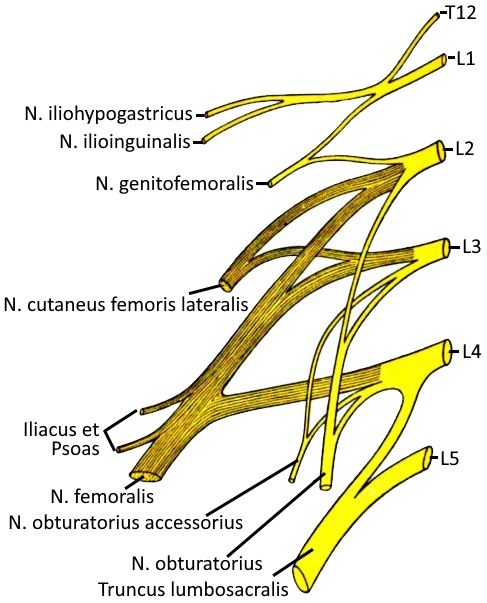
Lumbar plexus
As part of the PNS, the lumbar plexus is a nerve plexus consisting of anterior branches of the spinal nerves of the segments L1 to L3, additionally from parts of T12 and L4. The pelvic and leg nerves originate from the lumbar plexus and sacral plexus.
Sacral plexus
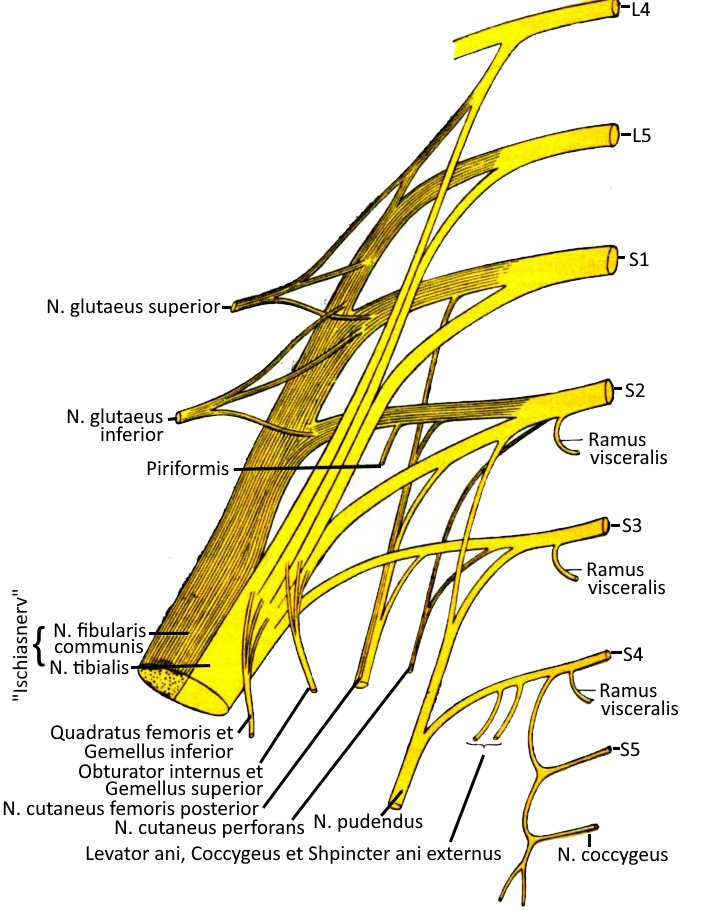
As part of the PNS, the lumbar plexus is a nerve plexus consisting of anterior branches of the spinal nerves of the segments L5 to S3, additionally from parts of L4 and S4. The nerves of the pelvis and legs originate from the sacral plexus and lumbar plexus.
Proprioception
Self-perception of the body independent of external sensors, i.e. the 5 senses. Proprioception makes it possible to perceive the position of the body in space and the position of the individual body parts in relation to each other, as well as the tension of the muscles and the force exerted. A distinction is made between
- Joint position sense: Sensation of the geometric position of the body and joints
- Sense of movement (kinethesia): continuous sensation of changes in the position of the body or body parts
- Sense of force and resistance: sensation of traction and pressure.
The sensors responsible for this are called proprioceptors:
- muscle spindle: fibres arranged in the muscle parallel to the muscle fibres, which record the proportional behaviour, i.e. the absolute length of a muscle, with static core fibres and the differential behaviour, i.e. the change in length, with the dynamic core fibres.
- tendon spindle (also: Golgi organ, Golgi tendon receptor): slow-adapting tension sensors located in the transition area between muscle and tendon fibres, basis of self-reflexes
- Sensitive receptors in joint capsule, ligaments, periosteum
In contrast to proprioception, visceroception enables the perception of internal organs. Both together are also referred to as interoception. Various proprioceptors are available for this purpose.
A distinction can be made between conscious proprioception, which is analysed during consciously and purposefully performed movements such as balancing or yoga postures, and unconscious proprioception, which is sufficient for mechanical activities such as walking or climbing stairs.
Proprioceptors
The receptors that enable proprioception are:
- Muscle spindle: fibres arranged in the muscle parallel to the muscle fibres, which record the proportional behaviour, i.e. the absolute length of a muscle, with static core fibres and the differential behaviour, i.e. the change in length, with the dynamic core fibres.
- Tendon spindle (also: Golgi organ, Golgi tendon receptor): slow-adapting tension sensors located in the transition area between muscle and tendon fibres, basis of the own reflexes
- Sensitive receptors in joint capsule, ligaments, periosteum
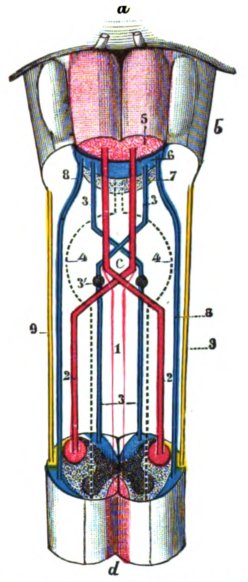
Pyramidal tract (tractus pyramidalis)
This is the pyramidal motor system of the voluntary motor system, which transmits the signals from the motor cortex to the voluntary muscles of the body. It is divided into
corticospinal tract: to the α-motoneurons of the peripheral musculature and
corticonuclear tract: to the motor cranial nerve nuclei
In the narrower sense, the pyramidal tract is only understood to be the corticospinal tract. In the pyramidal junction, 70-90% of the fibres cross to the contralateral side as tractus corticospinalis lateralis, the rest run as tractus corticospinalis anterior further to distal. The pyradial pathway primarily controls fine motor skills, while habitually honed gross motor skills are controlled by the extrapyramidal motor system. Both belong to the somatomotor system, i.e. they are somatic and motor fibres.
Pyramidal tract signs
Neurological symptoms that indicate damage to the pyramidal tract. Several are known for the lower extremity: Babinski reflex, Bechterew reflex, Chaddock reflex, Gordon reflex*, Oppenheim reflex, Mendel-Bechterew reflex, plantar muscle reflex, Rossolimo reflex*, Piotrowski reflex, dorsalis pedis reflex*, Strümpell reflex, but also some for the upper extremity: Gordon finger splay sign*, Trömner reflex*, Wartenberg reflex, non-exhaustible hand clonus, of which those marked with * are considered unsafe signs.
Disorders of the 1st motor neurone (UMN) usually lead to spastic paralysis, disorders of the 2nd motor neurone (LMN) to flaccid paralysis
spinal cord
(lat. medulla spinalis or medulla dorsalis) part of the central nervous system that runs in the spinal canal. The dorsal medulla ends caudally as conus medullaris (medullary cone) in the L1 / L2 area, where the nerves continue individually as cauda equina. The existence of a spinal cord is common to all vertebrates. It is enveloped by meninges (meninges, from outside to inside: dura mater, arachnoid, pia mater), which in turn are surrounded by cerebrospinal fluid (cerebrospinal fluid). Cranially, the spinal cord emerges from the medulla oblongata located above the foramen magnum. The spinal cord contains the grey and white matter, for a more detailed description see e.g. Wikipedia.
Spinal canal
The spinal canal, in which the spinal cord runs, and from which the spinal nerves emerge through the vertebral foramen.
Spinal nerve
The spinal nerves are the nerves that emerge in pairs (right/left) from the spinal cord. From their exit from the spinal cord, they are part of the PNS. The spinal nerves unite the anterior and posterior afferent and efferent nerve roots: Afferents with sensory information enter through the posterior nerve root (radix posterior), efferents with motor nerve fibres and partly also fibres of the n. vagus. vagus emerge through the anterior nerve root. pressure on a nerve root or the nerve root it contains. the spinal nerve that contains it can lead to nerve compression syndrome. The fusion of the nerve roots already occurs in the spinal canal.
Cerebrospinal fluid
Body fluid of the CNS formed by the coroid plexus as an ultrafiltrate of the blood serum, which circulates in a system of communicating cavities known as the cerebrospinal fluid space and serves the metabolism (nutrition and disposal) of the CNS in that the
interstitial fluid is derived from the cerebrospinal fluid. In analogy to the blood-brain barrier, there is a blood-cerebrospinal fluid barrier. In terms of disposal, the cerebrospinal fluid is the equivalent of the lymphatic system, which is not present in the brain. Lymphocytes make up the largest population with up to 3 / µl, monocytes are also rarely detectable. Proteins are also massively reduced compared to the blood serum (about a factor of 200), only glucose is present at 50-70% of the serum. The total amount circulating is approx. 150 ml. Of the daily production of 500-700 ml, the majority is absorbed at the granulationes arachnoidales and the nerve roots. Another part also flows along the cranial nerves and spinal nerves into the periphery.
Another function of the cerebrospinal fluid is to act as a kind of water cushion to mechanically protect the brain. In addition, the brain is hydrodynamically buoyed up by being embedded in water, which significantly reduces the pressure of the lower parts of the brain.
The CSF becomes diagnostically important if the number of leucocytes is increased, which indicates inflammation, or if erythrocytes appear in the CSF, which indicates a subarachnoid haemorrhage.
Parasympathetic tone
State of increased excitation of the parasympathetic nervous system.
Sympathetic tone
State of increased excitation of the sympathetic nervous system.
Vagus nerve (N. vagus)
The n. vagus is the antagonist of the sympathetic nerve and the most important part of the parasympathetic nerve. It is the 10th cranial nerve and is the only one that runs from the head to the torso, hence its name „wandering“. Among other things, it controls digestion and calms the heart, see the effects of the parasympathetic nervous system and the heart rate variability (HRV).
vagotone
State of increased excitation of the n. vagus.
Pain.
Pain is a complex subjective sensory perception which, as an acute event, has the character of a warning and guidance signal and can range in intensity from unpleasant to unbearable. As chronic pain, it has lost the character of a warning signal and is now seen and treated as an independent clinical picture (chronic pain syndrome). (Wikipedia)
IASP (International Association for the Study of Pain): Pain is an unpleasant sensory or emotional experience that is associated with actual or potential tissue damage, or is described in terms of such damage.
ICD (International Statistical Classification of Diseases and Related Health Problems; currently ICD-10, from 2022: ICD-11, WHO classification system for medical diagnoses): Pain is a complex sensory sensation caused by the excitation of pain receptors. Pain often occurs with the involvement of other senses (pressure and temperature), and the mental state also plays a role. A distinction is made between different pain qualities such as burning, drilling, throbbing or stabbing pain.
In general, pain is regarded as a biological alarm signal and represents a protective function of the body. Pain is an important symptom in medicine, but can represent an independent clinical picture, especially in psychosomatic illnesses.
Nociceptor
Free nerve endings in tissues that absorb pain are called nociceptors. They are found in the skin but also in almost every other tissue. They react to various conditions, such as mechanical (pressure), chemical and thermal. Signals from the nociceptors usually, but not always, lead to a sensation of pain. Organic pain, on the other hand, is always based on signals from nociceptors. See Nociceptor pain
Forms of pain
Nociceptor pain
Nociceptors (free nerve endings) react e.g. chemically, mechanically, thermally and show hardly any adaptation in contrast to receptors
– Somatic (affecting the body) Nociceptor pain is caused by irritation of the nociceptors of the skin, mucous membranes, bones, skeletal muscles, ligaments, tendons, muscles, connective tissue, hollow organs, etc. and is sharply limited, sharp and severe.It is sharply defined, easy to localise and stinging to burning, permanent or swelling and subsiding, and the person usually reacts by avoiding movement/keeping still. There are perceptible differences between surface pain (skin) and deep pain. Next: First surface pain (short, bright, sharp, easy to localise, pithy fibres), second surface pain (diffuse, dull, burning, slowly subsiding, pithless fibres, corresponds neurophysiologically to deep pain)
Visceral (affecting the viscera) nociceptor pain when nociceptors in the internal organs of the pelvis, abdomen and chest are stimulated. This pain is pressing, pulling, dull, burning, undulating, drilling; continuous or colicky, cramp-like, undulating; diffuse, not easily localised. Examples: Stretching of hollow organs, spasms of smooth muscles, inflammation, circulatory disorders
Neuropathic/neurogenic pain (irritation/compression/damage of peripheral nerves or compression of nerve roots in the area of the spine)
a. short/acute: cutting, stabbing and attacking, shooting in like a flash, not easy to localise, perceived localisation possibly distal to the actual event.
b. Permanent pain: burning or drilling
Examples: Amputation phantom pain, herpes zoster neuralgia, polyneuropathy.
Pain due to functional disorders
e.g. due to vascular dysregulation, muscle tension, hard tension, also psychosomatic pain. This pain is also referred to as functional pain.
Chronic pain syndromes (>= 6 months)
1. Inflammatory pain (often chemically induced)
2. Spastic (excessive contraction of the musculature)
3. Nerve pain (irritation of nerves of the CNS/PNS)
4. Misregulation pain (dysfunction of the motor system, e.g. overtension of muscles, or of the sympathetic NS, e.g. ischaemia due to vasospasm, misregulation of neurotransmitters, e.g. migraine)
5. psychomotor pain (expression of psychomotor states)
The NS consists of a part subject to arbitrariness („animales“ NS) and an „autonomic“ or „vegetative“ NS not primarily subject to it with the three areas
– sympathetic NS (energy discharge, action, „ergotropic“)
– parasympathetic NS (energy storage, recovery, build-up, „trophotropic“)
– intramural (walls of the hollow organ yarns)
Muscle spindle, muscle receptorsoren (stretch receptors, mechanoreceptors)
The muscle spindle is an intramuscular sensory organ (proprioceptor) that detects the relative muscle length, which corresponds to a measurement of the
sarcomere length. They record proportional and differential changes, i.e. changes in length and their acceleration. The muscle spindles largely protect the muscle from overstretching by means of negative feedback, in which a signal originating from the muscle spindle reduces the contraction of the muscle by the UMN motoneuron. In addition, the muscle spindles are responsible for the own reflexes, in that the signal they send out leads to a twitching of the muscle during the reflex test in the way just described. Muscle spindles are 5-10 striated fascia surrounding muscle fibres, usually 1-3 mm long in humans. In human quadriceps there are around 500-1000 muscle spindles up to 10 mm long. The number of muscle spindles determines how finely the force development of a muscle can be dosed. The non-contractile centre of the muscle spindles is surrounded by afferent sensitive fibres, which respond to a stretching of the muscle with an action potential, which is transmitted via the spinal nerve to the grey matter of the posterior horn and via a synapse to the alpha-motoneuron. The Renshaw cell embedded in the control circuit causes a short-term inhibition of contraction. The contractile border areas of the muscle spindle have a gamma spindle loop that is connected to the gamma motoneurons via motor nerve fibres and. If the gamma spindle loop generates an action potential, this leads to a contraction of the muscle, during which the non-contractile middle section, which is responsible for inhibition, relaxes and reduces its signal. This happens in a control loop until the middle part no longer perceives any stretching. The spindles are partly responsible for ensuring that the muscle produces and maintains a constant tonus, which also allows a joint to be held in a constant position.
Pain history (short form)
– Localisation ? Extension ? Punctiform, circular, flat, elongated ? narrow, broad ? Direction ?
– Quality/type (see below) ? Change ?
– Rest pain / Motion pain / Exertion pain ?
– Time ? Duration ? First occurrence ? possible triggers ?
– Severity ? Course ? Modalities (influencing factors) such as time of day, food intake, exercise, other ?
– Accompanying symptoms ?
– What improves, worsens ?
– Important for us: muscular pain or non-muscular pain ?
Pain qualities
Pain can be categorised (without claiming to be exhaustive):
1. sensory
-radiating
-boring
-burning
-muffled
-pressing
-thin
-bright
-fein
-großflächig
-klopfend
-kolikartig
-krampfartig
-peitschend
-sudden (acute)
-pulse-synchronous
-tearing
-creeping (increasing)
-cutting
-piercing
-wehenartig
-wellenförmig
-klopfend
-pochend
-ziehend
-zuckend
-heiß
Examples:
stinging: pleurisy
burning: skin abrasion
pulling: Back pain
whipping: nerve pain, shingles
destroying: heart attack
drilling: Tumours, also bone tumours
spasmodic/colicky: renal/gallbladder colic
painful, cramp-like: menses
constricting: angina pectoris
pressing: swelling, inflammation
2. affective(without claim to completeness)
-beklemmend
-vernichtend
-heftig
-nervend
-lähmend
-schrecklich
-marternd
-quälend
Furthermore, they can vary, this is referred to as:
Pain modalities
Typical pain modalities include:
- Night/day
- rest pain /movement pain -stress pain
- after excitement
- depending on the situation
- after the meal/before the meal (sober)
- depending on the season/weather
- Breath-dependent
- improving, worsening
- Triggering pain
Pain localisations
Irrespective of the various specific locations (localisation), extensions can be described, such as
punctual
surface
elongated or transversely extended or otherwise orientated (direction ? thickness ?)
superficial (superficial) or deep (profound, then: how deep)
Characteristic pain
Start-up pain (running-in pain, running-out pain)
Pain that occurs at the start of an activity, movement or task and subsides as it progresses. This type of pain is typical of arthrosis and arthritis, but also other degenerative joint changes, especially of the hip joints and knee joints. In the case of arthrosis, the disease progresses over the years progressively, and thus the initial initial pain often turns into persistent pain on exertion and later also rest pain. In arthrosis, both are particularly pronounced in arthritic episodes.
Stress-induced pain
the pain that is triggered by strain on stress-induced pain parts of the movement system.
Painfulness on exertion
The property of a muscle, its tendon or another structure of the musculoskeletal system to exhibit pain on exertion, i.e. to show pain under stress, which has a quality other than „exertion“ or „stretch sensation“, i.e. is of a different origin than from the proprioceptors Golgi tendon organ or muscle spindle.
The load can be your own body weight or an external weight. The load from your own body weight can be, for example, a squat, climbing stairs or the muscle work required for fast walking or running. Exertional pain mainly affects the musculoskeletal system and is usually, but not always, caused there.
Pain in motion
Pain that occurs during active or passive movement without the weight of the body or an external weight. The pain on movement can be divided into active pain (the movement is performed by the person being examined) and passive pain (the movement is performed by an examiner). Motion pain is typical for specific parts of the musculoskeletal system such as muscles and joints. The back, shoulder joint and knee joint are frequently affected. The causes of pain on movement can be varied: degenerative diseases of the musculoskeletal system, fractures, ruptures, fibromyalgia, polyneuropathy, various diseases of the spine or inflammation.
Painfulness of movement
The ability of a part of the movement apparatus (rarely: internal organs) to trigger movement pain through actively or passively induced movement, even without load.
Stretching pain
Pain sensation triggered by stretching, i.e. moving a muscle to borderline large or increasingly larger sarcomere lengths, typically localisable by radiating in the direction of the muscle course. Physiologically, stretch pain is NRS 0 to 10 scalable and subsides significantly within seconds after the application of force to the muscle with only brief reverberation. The proprioceptors that report the stretch pain are the muscle spindles.
Stretch pain
Stretch pain refers to the ability of a part of the movement apparatus (usually the muscle and its tendon) to exhibit pain when the muscle is actively (by the application of force by the antagonists) or passively induced to stretch, that goes beyond the physiological level of stretching pain or shows a different pain quality. Various disorders of the muscle (e.g. ruptures, strains, muscle bruises) or tendon (e.g. strains).e.g. insertional tendinopathies, tendovaginitis) can be associated with painful stretching.
Pressure pain
Pain that is triggered by external intervention such as palpation or other mechanical action on tissue that exhibits pressure tenderness (pressure tenderness). The sensation of pain is usually dependent on the intensity of the pressure. This pressure can also be pressure caused by clothing or footwear. The pressure pain may subside immediately when the external pressure is stopped or may „reverberate“ a little (post-loading pain). pressure pain very often indicates inflammation. It must be differentiated conceptually from „pressing pain“, which indicates increased tension in a tissue, is also often caused by inflammation, but is not triggered by deliberate pressure from the outside, but is present without any intervention.
Pressure pain / pressure dolence
pressure pain or pressure dolence refers to the current ability of a body part to produce pain sensation (pressure pain) in response to external pressure. In most cases, the quantity of the transmitted pain sensation („intensity“) is monotonically dependent on the amount of pressure exerted. The resulting pain is referred to as pressure pain. It should not be confused with tension pain, which occurs without external intervention such as palpation.
functional pain
Functional pain is not a clearly defined term. As a rule, it refers to pain without a demonstrable non-clinical correlate, such as evidence of inflammation, fracture, a bursitis, a muscle fibre tear, a arthritis or other manifest, verifiable disorders. Functional pain is often also understood to mean pain caused by disorders that are detectable by appropriate means but are only slightly noticeable, such as the degenerative symptoms of insertional tendopathy. Classic functional pain includes muscle tension, but also changes in the capsule or ligament tension of the joints. The conceptual counterpart to functional pain is structural pain.
ischaemic pain (ischaemic pain)
Ischaemia is known from myocardial infarction or its precursor, angina pectoris, but also occurs elsewhere, for example as paVK in the form of intermittent claudication (intermittent claudication), which makes even medium walking distances impossible due to pain. Bloodless apoplexy is also ischaemia, although it does not lead to pain (silent ischaemia).
Tapping pain
Pain that occurs beyond the physiological level due to tapping on an anatomical structure, such as an inflamed area or a „diseased tooth“.
Inguinal pain
Functional pain or structural pain in the groin region. The functional pain is usually caused by insertional tendopathies of the following muscles:
This includes muscles from different functionalities, essentially: Abdominal muscles, hip flexors, adductor muscles. In contrast, structural pain is based on manifest structural disorders such as joint blockages (usually of the ISG (ISG-Blockade), subordinate of the hip joint), arthrosis, especially of the hip joint (coxarthrosis), secondary to the ISG, disorders of the pubic symphysis, fractures and stress fractures, in younger people, disorders of the hip joint such as Morbus Perthes or Morbus van Neck. It is also not uncommon to find disorders in adults that can be traced back to disorders of this type that were untreated or inadequately treated in childhood/adolescence, to dysplasias and incongruences.
The sports history is often informative and indicative in groin pain. The symptoms are often caused by or during football, running sports (especially sprinting and hurdling), and less frequently during walking, jumping and martial arts. Acute injuries are often muscle fibre tears or tendon tears (or tears). Chronic disorders are often overuse syndromes, such as insertion tendopathies, joint wear and tear (arthrosis) often due to minor disorders of the movement apparatus such as axial misalignments, foot deformities, muscular dysbalances (e.g. disorders or weaknesses of the hips).e.g. disorders or weaknesses of the hip muscles). In the clinical examination, it should not be overlooked that information on the localisation of pain is not always reliable, as e.g. disorders of the hip joint can project in the direction of the knee or the ISG in the direction of the groin.
Palpation often reveals pressure tenderness, which is indicative of a diagnosis. In functional tests (page comparison!), painful stretching or movement restrictions associated with joint pain may be found. Movements performed against resistance usually indicate painful strain due to insertion tendopathies, if the pain is close to insertion, otherwise disorders of the muscle itself, but this does not fall within the scope of the disorder groin pain. Disruption and reduction of exorotation can be muscle-related (contracture/shortening), but also associated with disorders of the ISG if pain is indicated. Restrictions of endorotation, on the other hand, are usually articular in origin and are based on arthrosis (usually coxarthrosis), arthritis or capsulitis. If the movements that cause pain or restrictions are more complex, further investigation is required; the cause is often ultimately found in the hip joint. Leg length discrepancies are always candidates (among other causes) for causing groin pain. If they are variable (sometimes present, sometimes not), this is often due to a disorder of the ISG.
Afterload pain (pain reverberation)
If a pain caused by exertion does not subside immediately after the end of the exertion, this is referred to as post-exertion pain, also known colloquially as „reverberation“ of the pain.
Night pain
Night pain means pain that only occurs at night. It is usually joint pain caused by arthritis, arthrosis and diseases of the rheumatic type, in particular rheumatoid arthritis. Generally, night pain of this type often also occurs during the day when resting (for a sufficiently long time). During pregnancy, night pains also occur from time to time, mainly due to fluid accumulation in the lymph vessels of the extremities (especially the legs). Several litres of fluid can be stored, which leads to tension pain and pressure pain in the support area.
Pseudoradicular pain
Nonspecific, localised pain with radiation („referred pain“) in the direction of an extremity similar to radicular pain symptoms, but without their efferent or afferent neurological deficits (in the area of innervation or sensitivity), as the spinal nerve itself is not impaired in its function. Pseudoradicular pain occurs, for example, as a result of facet syndrome, muscular or joint disorders, ISG blockages.
Pulse-synchronised pain (throbbing, knocking)
Pulse-synchronised pain is most common as a knocking or throbbing, as in a throbbing headache or throbbing toothache. Durafistula disorders (pulse-synchronised tinnitus) also exhibit this characteristic. In the musculoskeletal system, pulse-synchronised pain is found less frequently, mainly in connection with inflammation, when the arthritic supply leads to pulse-synchronised increases in pressure in an already tense tissue.
The term tapping pain should not be confused with knocking pain, i.e. the pain that is triggered by tapping on a structure that causes knocking pain.
Radicular pain
Pain caused by irritation (e.g. due to pressure) of a nerve root, as occurs in nerve root compression syndrome (radiculopathy). It is usually caused by intervertebral disc events, osteophytes or inflammation. Typically, the pain projects to the dermatome of a nerve root. Radicular pain needs to be clarified and often requires treatment. The compression of nerves can lead to their atrophy, but also to atrophy of the supplied muscles. The term neuroradicular is also used synonymously. See the difference to pseudoradicular here.
rest pain
Pain that occurs (also or only) at rest without active or passive movement and without strain. Parts of the body that are painful at rest are usually also painful during movement. Pain at rest occurs in the movement apparatus frequently in arthritis, arthritis, insertion tendopathies, fractures, nerve constriction syndromes (carpal tunnel syndrome, cubilar tunnel syndrome, tarsal tunnel syndrome), cervical spine syndrome. Other types of rest pain include ischaemic, cardiovascular and neurological rest pain. Rest pain can be of varying quality, such as tension pain (inflammation) or drilling pain (bone events).
Tension pain
Tension pain is pain that occurs due to increased tissue tension in tissues that have receptors to register this type of change in physiological state and transmit it to the brain. Tension pain must not be confused with pressure pain (pressure dolence) or the pain caused by palpation resulting from pressure pain. A classic example of clear tension pain with additional pressure pain (pressure dolence) is herpes labialis: Even without touch, there is a painful sensation of tension, which can be further increased by touch or pressure. The pain quality of tension pain is generally described as „pressing“ or „tense“, as is the case with pressure pain.
Structural pain
Structural pain, like its conceptual opposite, functional pain, is not sufficiently clearly defined. Structural pain is pain that is characterised by a (often: not too complex) detectable disorder such as an inflammation, a hernia, a burtisis, a muscle fibre tear, a arthrosis.
Growing pains
Growing pains are leg-emphasised nocturnal pains or pains that begin in the evening and usually occur bilaterally, less frequently also in the upper extremity. The sensory pain quality is usually described as pulling, burning or stabbing. They are usually harmless and affect up to 30% of 3-12 year olds. The aetiology and pathogenesis are still unclear, changes in the blood count are not observed, but a familial disposition is.
Massages, cooling, massaging and stretching of the local muscles are recommended for relief, as well as NSAIDs if necessary.
